What are CPGs and CDSTs ?
Clinical Practice Guidelines (CPGs) and Clinical Decision Support Tools (CDST) are medical documents that serve different purposes.
CPG are broad guidelines based on evidence to guide treatment of specific health conditions, typically in the form of written documents, including best practice recommendations and sometimes flowcharts. They provide a general framework but allow for adjustments based on individual patient circumstances, and have to be regularly updated to reflect the latest medical research and practices.
CDST are meant to assist healthcare providers in making patient-specific decisions, either in real-time or during the planning phase of treatment. They exist as written documents, standalone applications, or web-based tools. CDST can include a variety of support mechanisms like diagnostic assistance, treatment options, and patient-specific alerts or reminders.
Producing CPGs and Clinical Decision Support Tools (CDST) is one of the main goals of ERNs. The goal can be reached by developing new recommendations, and/or by endorsing existing national CPGs.
Developing CPGs for rare or low-prevalence diseases differs from that of more common diseases due to often limited knowledge on their natural history and health care needs : CPGs on rare disorders can often not or scarcely be based on literature as often no sufficient data are available. All CPGs rest on both the limited literature and a consensus of experts, based on the experience of healthcare professionals, scientists, and representatives of patients; however, CPGs for rare disorders may rely a bit more heavily on the consensus of aforementioned stakeholders. The discussions between these contributors, with the aim to come to a single opinion on the main topics if at all possible, form the core characteristic of consensus.
The procedure applied by ITHACA for CPG development follows several methodological steps that are summarized below and was discussed in a dedicated Webinar.
1. Forming the infrastructure
The first step is to form a core group with an appointed chair, and a consortium. The core group comes together to oversee the project and can make decisions on the process of the project. Medical and non-medical experts experienced in the diagnosis, clinical management and care of individuals with a specific condition should be involved in the consortium (e.g. clinical geneticist, paediatricians, cardiologists, pulmonologists, neurologists, psychologists, psychiatrists, rehabilitation specialists, ophthalmologists, ear-nose-throat specialists, social workers etc…). Patient representatives should also be engaged in the consortium, for which ERN-ITHACA can provide support. Also, a methodologist should be part of the consortium (preferably of the core group). It is the responsibility of the chair to involve appropriate experts as relevant to the CPG and to ensure that the consortium keeps its competency until the end; consortium member should be aware that CPG development may be a long process (at least 18 months). The consortium may exist of several working groups, each dedicated on a specific topic within the CPG. Each working group should have an appointed leader. ERN-ITHACA may provide support related to project management and organization and/or methodological advice. All consortium members should have filled out a statement on their Conflicts of Interest.
2. Framework, analysis of clinical issues
Topics that will be covered by the CPG should be selected. Working group leaders are responsible for entrusting members of their working groups with specific tasks (eg. literature search, formulation of texts) and follow-up on their progress. This should be done via correspondence and digital meetings.
There are two types of meetings:
- Working group-meetings: members of a working group organize a meeting,
- consortium-meetings: everyone taking part in the CPG development from all working groups are invited.
3. Prioritization of clinical issues (PICOs)
Identification and prioritization of health issues (preferably defined within each working group) that are of most interest in patients and caregivers – these are the core questions of the CPG that are to be addressed. PICO questions (acronym for Population, Intervention, Comparison, and Outcome) should be named together with patient representatives within each working group. Prioritization is key: not all clinical issues that are part of a specific condition can be addressed. Prioritization should be based on expertise of patient representatives and clinicians, and the fundamental question of whether recommendations will improve quality of care differences in the EU. A methodology for prioritization may be used; available options are group discussion, a survey and a prioritization tool.
4. Literature search and selection
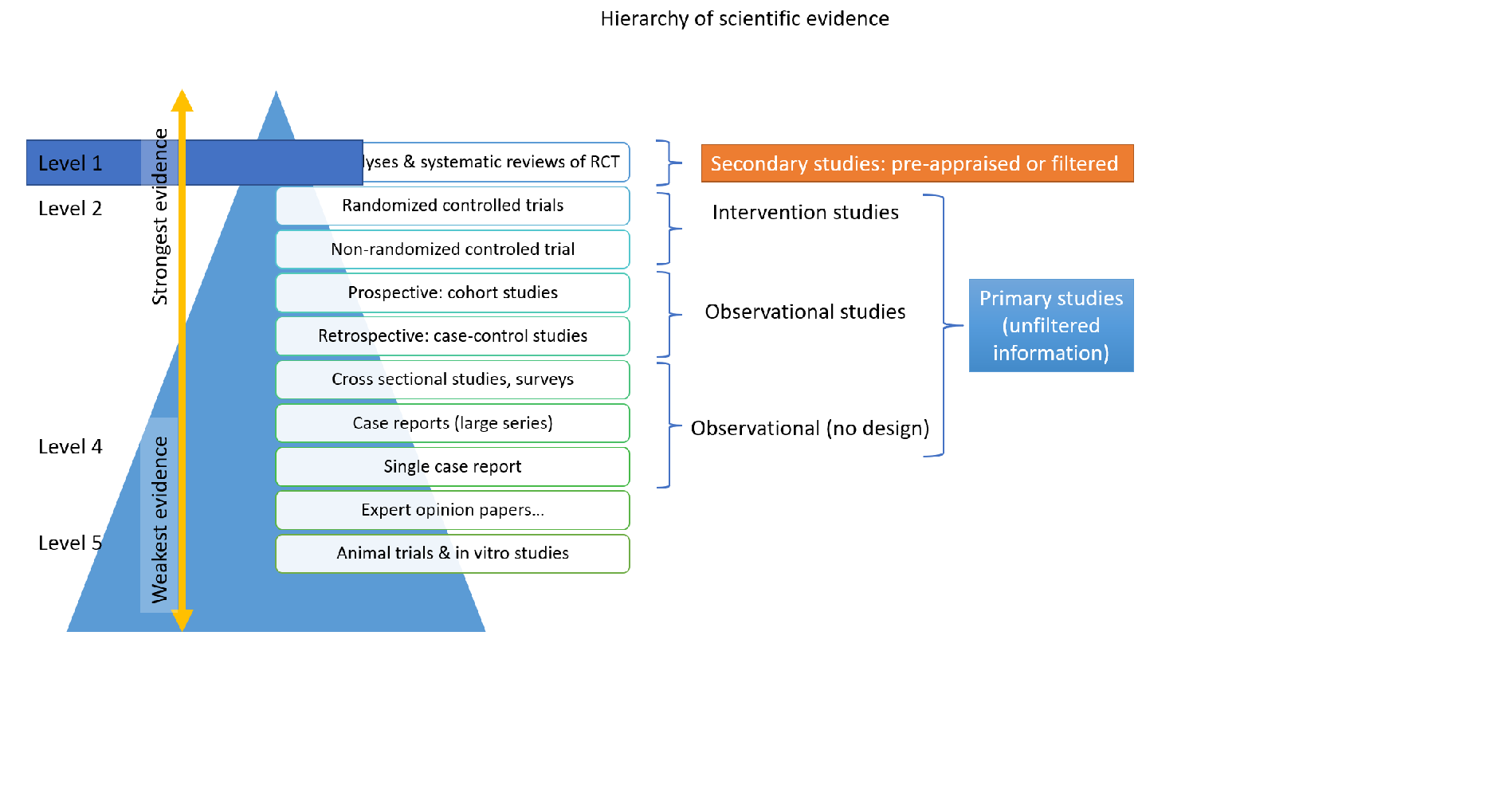
Literature search should be thorough and oriented along the PICOs. Randomized controlled trials or meta-analyses are scarce on rare diseases, if at all available. Members of the working groups should perform literature search based on agreed keywords. Selected literature should be listed and made available in a common library or drive to all members of the working group(s). In rare diseases, literature reports on even a single patient are valuable, even those should be taken into account with careful interpretation of conclusions. Preliminary literature search may generate new PICOs.
Review of existing disorder-specific CPG (if any) is a part of the work. Existing transversal GPG targeting a clinical situation (and not a specific clinical diagnosis) must be searched and evaluated for their relevance in a specific diagnostic context.
5. Literature summary, GRADE analysis
One or more members of the working groups should survey the available literature, summarize it either in a few key sentences or organize the data derived from the literature (Excel sheet) to facilitate use, based on field of interest. The GRADE method can be applied to evaluate the quality of the evidence. Grading should be done per PICO.
6. Recommendations
Recommendations should contain the key messages of chapters written per working group, and a recommended procedure on a specific health issue in one or two sentences and be placed in each chapter of the CPG either as highlighted text or a text box. They are based on both the summary of the literature and the expert opinion of all consortium members. Recommendations should be stand-alone sentences. They should be formulated in a way that they are applicable in countries with diverse levels and possibilities of health care (international standardization); for instance, both minimum care and optimal care recommendations may be specified. Wording should be carefully balanced in line with literature, expert opinion and support decision-making. The CPG should serve as an advisory tool and not as a protocol without further considerations.
7. Consensus meeting, round of comments
A face-to-face consensus meeting with open discussions and coming to a consensus is mandatory. Such a personal meeting improves the quality of the CPG. Consensus means discussing all ins and outs of each topic, not just by the specialists on that topic, but also by those with less experience who often ask just those questions a specialist is no longer considering, and especially also incorporating the opinion of psychologists and other allied health care professionals, and representatives of lay organisations who are the best positioned individuals to consider the consequences for the well-being of the patients and the consequences of a suggested management scheme for them.
After every working group has finished writing their texts, the chair should synthesize, edit and harmonize the texts of the working groups in one common text file with the recommendations. When this is ready, a face-to-face meeting or a hybrid meeting with a minimum of two experts standing for their working group plus a minimum of one patient representative per working group should be organized. In CPGs supported by the ERN, costs of travel and accommodation of clinical experts/patient representatives who gather for a face-to-face meeting needs to be agreed upon with the project managers. A minimum of two days’ time is necessary for a face to face meeting. On day one, the whole text of the CPG recommendations is read out loud by the chair, chapter by chapter, and open discussions, major or minor corrections regarding content, formulation, phrasing, and other adjustments can be done. On day two, consensus should be reached regarding every recommendation.
8. Voting
Recommendations should be voted on, in a democratic, preferably anonymized fashion. Each working group should be represented equally in the voting of each chapter. Working group members should not abstain from voting on other working groups’ text and recommendations unless they indeed lack general knowledge in a specific field; preferably members of the working groups vote on their own and other working groups’ texts and recommendations.
9. Deliverables
The following deliverables are developed:
(1) Scientific, peer-reviewed publication or related publication bundle, preferably in a peer-reviewed, open access journal;
(2) Full version of CPG available on ERN ITHACA’s website;
(3) Healthcare provider summary, including all recommendations and surveillance scheme where applicable;
(4) Lay summary, including all recommendations at least in English and in as many European languages as possible.
When possible and needed, a research agenda may be formulated based on the identified literature gaps
Further notes
For peer-reviewed publications of CPGs supported by ERN-ITHACA, the ERN ITHACA Guideline Working Group is mentioned in the author list of the journal article.
For the endorsement procedure of already existing CPGs, contact the ERN-ITHACA Guideline Working Group.
Further readings
For the extended version of the European Reference Network Handbooks on Clinical practice guidelines and clinical decision support tools, consult this page: ERN GUIDELINES Methodological Handbook .
This methodology is based on the standards of the AGREE II instrument (Appraisal of Guidelines for REsearch and Evaluation II). See also: https://www.agreetrust.org/agree-ii/
For more information on the GRADE approach, see this methodological summary (Understanding GRADE: an introduction – Goldet – 2013 – Journal of Evidence-Based Medicine – Wiley Online Library ) For in depth approach, visit GRADE handbook.
